Bassam Tabshouri*, Binseng Wang§
*HTMA, Lebanon tabshb@gmail.com
§Sodexo HTM, USA binseng@alum.mit.edu
Abstract #
The practice of “critical appraisal” in medicine started in 1981 and was later named as “evidence-based medicine” (EBM) ranked seventh among the 15 most important milestones that shaped modern medicine in a poll by the British Medical Journal in 2007 [1]. Drawing from the principles of EBM, this paper describes a new approach for the maintenance of medical equipment: Evidence-Based Maintenance. Both EBM’s have much deeper roots, i.e., the scientific method that started in the Renaissance (16th century) and first revolutionized physics and then chemistry and, later, biology. Evidence-Based Maintenance is based on the same principle as Evidence-Based Medicine, i.e., use clinical outcomes to evaluate and improve the care of patients. The only difference is that the “patient” is the medical equipment in the latter [2].
Keywords: Evidence-Based, Maintenance, Medical Equipment.
1 Introduction #
In addition to ensuring safety, the main aim of the classical approach to medical equipment maintenance within the healthcare delivery organization (HDO) is to reduce maintenance costs and minimize capital investment (CapEx). This calls for the clinical engineering (CE) professionals to maintain equipment as recommended by the manufacturer as mandated by Joint Commission International (JCI) and manage the equipment lifecycle within the HDO.
However, CapEx is only ~20% of total cost of ownership (TCO), while the maintenance cost is only ~1% of total hospital operating expense (OpEx). Thus, CE professionals need to look beyond equipment safety and reliability in order to contribute more effectively to the care of patients not only within the HDO but also in the entire continuum of care, including ambulatory sites, clinics, and homecare.
Before explaining the EBM methodology, it is worthwhile to understand how CE professionals have been maintaining medical equipment for the last few decades and why there is a need to adopt a new approach.
2 Classical Approaches to Medical Equipment Maintenance #
2.1 Risk-Based Criteria (the Fennigkoh & Smith model) #
This approach assigns an Equipment Management (EM) number for every piece of equipment to determine the maintenance strategy as shown in Table 1 [3].

Where PM is the abbreviation for preventive maintenance (later recharacterized as scheduled maintenance).
While initially useful to stop performing PM on every piece of equipment with the same frequency and tasks, the Risk-Based Criteria actually only considers risk severity, without contemplating risk probability as recommended by the ISO 14971 standard, which defines risk as a combination of probability & severity [of harm]. A visualization of failure probability is provided in figure 1, which shows that while multiple layers of defense are usually deployed, each one has some gaps and holes that can allow failures to go through. The failures related to human actions are called “active failures,” while those related to organizational processes are called “latent conditions.”
| CRITERA | CATEGORY | SUBGROUP | NUMERICAL VALUE | ||||
| Function | Therapeutic | Life Support | 10 | ||||
| Surgical and intensive care | 9 | ||||||
| Physical Therapy | 8 | ||||||
| Diagnostic | Surgical and intensive care | 7 | |||||
| Additionaldiagnostic | Physiological | monitoring | and | 6 | |||
| Analytical | Analytical Laboratory | 5 | |||||
| Laboratory accessories | 4 | ||||||
| Computer and related | 3 | ||||||
| Miscellaneous | Patient related | 2 | |||||
| Physical Risk | Patient death | 5 | |||||
| Patient or operator injury | 4 | ||||||
| Inappropriate therapy | 3 | ||||||
| No significant risks | 2 | ||||||
| Maintenance Requirements | Extensive | 5 | |||||
| Average | 3 | ||||||
| Minimal | 1 | ||||||
Table 1: Risk-Based Criteria for determination of maintenance strategy
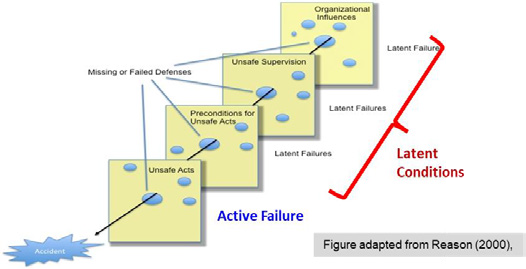
Figure 1: The Swiss-cheese model of risk
2.2 Reliability-Centered Maintenance (RCM) #
RCM is defined as “a process used to determine what must be done to ensure that any physical asset continues to do what its users want it do to in its present operating context” [4]. This approach improves asset performance and is able to contain and even reduce the cost of maintenance. It has three basic elements: Planning, Implementation, and Evaluation.
- Planning
- Asset selection
- Characterization of function & failure patterns
- Failure mode and effect analysis (FMEA)
- Decision process
- Develop performance measures
- Define maintenance schedules & work instructions
- Staff training
- Implementation
- Implement adopted strategies
- Maintenance data collection
- Evaluation
- Evaluate maintenance performance
- Use evaluation results to revise & update strategies
RCM uses a decision process to determine the best maintenance strategy according to an analysis of the failure modes and effects as shown in figure 2.
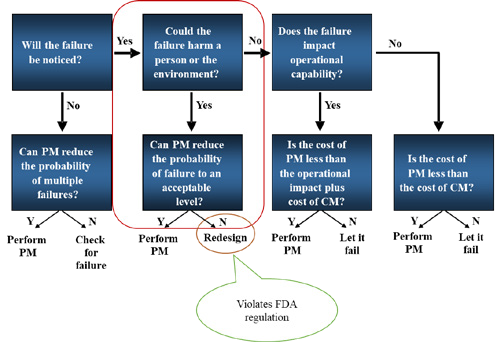
Figure 2: RCM Decision Process (Adapted from [4])
While RCM has been very successful in many industries, e.g., aviation, manufacturing, and electricity generation and distribution, it has significant challenges for application to medical equipment due to:
- Failure mode and effect analysis (FMEA) is often not possible because the manufacturers do not share the software embedded into the medical equipment.
- When PM cannot reduce probability of failure, RCM recommends redesigning the device. However, this is almost always in violation of regulations of national medical device authority, e.g., the Food and Drug Administration (FDA) in the USA.
- Without manufacturers’ assistance, it is difficult to define maintenance schedules & work instructions.
3 Evidence-Based Maintenance – EBM #
EBM is a continual improvement process that analyzes the effectiveness of maintenance resources, structure and processes deployed in comparison to outcomes achieved previously or elsewhere and makes necessary adjustments to maintenance planning and implementation [2].
The EBM methodology is based on the analyses of maintenance data collected, not only from an individual hospital, but also hundreds of others whenever those are available. The aim is to improve capital planning, increase utilization, reduce unnecessary maintenance, delay premature replacement, enhance safety, and protect against cyberattacks and thus increase productivity and revenue.
3.1 Maintenance Strategy Selection (Planning) #
Medical equipment maintenance is composed of two type of maintenance activities:
- Scheduled Maintenance (SM), which is composed of
- Preventive Maintenance (PM): replacement of parts with predictable deterioration (e.g., O2 cell or rubber gaskets), and
- Safety & Performance Inspection (SPI): detection of hidden and potential failures.
- Corrective Maintenance (CM), which aims at restoring the equipment to its original safety and performance specifications.
SM should be planned as a function of the technology used its construction (e.g., mechanical, pneumatic, and/or chemical) and not as a function of the mission criticality or the risk of the device to individual patients. This is because more frequent or elaborate maintenance cannot increase safety or reliability, unless there are failures that can be prevented by PM or detected by SPI. As most CE professionals know by their experience, maintenance needs have changed significantly with technology evolution. For example, changing from CRT tubes to LED panels in patient monitors and from X-ray films to digital X-ray detectors have dictated completely the proper maintenance methods for those types of medical equipment.
In industrialized countries CM of the equipment is done as needed if the cost of repair is below a certain percentage of replacement cost (e.g., 25%); otherwise, it will be replaced. However, this may not be easy now with budget problems or in other countries. Examples of such equipment are patient monitors based on solid- state electronics and pulse oximeters.
The references used for the determination of SM frequency and activities are the manufacturer’s service manual (if available), the published recommendations by professional organizations, the past experience of individual hospitals, and the shared experience among hospitals.
3.2 Evaluation of the Maintenance Strategy EB #
In EB Medicine, a drug or medical procedure must be evaluated for its safety and effectiveness before it can be marketed. In EB Maintenance, we also need to evaluate the maintenance strategy for its safety and effectiveness. To measure effectiveness, we use reliability because the availability of the equipment for use whenever needed tells us how effective the maintenance strategy was adopted.
In other words, a maintenance strategy should be evaluated for its reduction of equipment malfunctions that can negatively affect patients and clinical users (safety) and for the equipment availability when needed (reliability = effectiveness). These two dimensions are like the two sides of the same coin. It is useless to have a safe equipment that is not reliable or to have a reliable piece of equipment that is not safe.
Like EB Medicine, EB Maintenance tries to find ways to address the root causes of the failures (diseases in medicine) and uses the outcomes to determine which maintenance strategy works best. Root Cause Analysis (RCA) of failures is needed to know why a piece of equipment failed and then, determine what can and needs to be done to prevent it. Full RCA is time consuming but is necessary when a patient injury occurred. On the other hand, a simplified RCA can be used for routine assessment of equipment failures. Thus, a limited set of failure causes, called Failure Cause Codes (FCCs), have been developed and deployed. The FCCs are show on Table 2.
Using the FCCs, one can evaluate the maintenance strategy adopted (i.e., maintenance activities and respective frequencies) for safety and reliability as described below [2].
3.2.1 Safety Evaluation Using EBM
First, record all patient incidents (including “near misses”) involving medical equipment failures. Next, perform a root-cause analysis (RCA) and assign the appropriate FCC. Then focus on incidents related to these FCCs: service induced failure (SIF), hidden failure (HF), potential failure (PF) and preventable and predictive
failure PPF, as these causes are related to maintenance. Finally, determine whether these failure causes are related to individual actions or maintenance strategy, i.e.:
a) “unsafe acts” (or “active failures”) committed by individual staff (employed by hospital, original equipment manufacturer (OEM), or third party), e.g., lapses or slips.
b) “latent conditions” created by the organization due to oversight or deliberate violation of regulations, codes or standards.
How the results of safety evaluation are used to improve maintenance is described later in section 3.3.
| Code | Failure Cause Description | SM/CM |
| NPF | No problem found (or the reported problem was not duplicated). | both |
| UPF | Unpreventable failure, typically caused by normal wear and tear but is unpredictable. | CM |
| ACC | Accessory failure, excluding batteries, typically caused by normal wear and tear. | both |
| BATT | Battery failure, i.e., battery(ies) failed before the scheduled replacement time. Does not include scheduled replacement of batteries. | both |
| NET | Failure in or caused by network, while the equipment itself is working without problems. Applicable only to networked equipment. | both |
| USE | Failures induced by use, e.g., abuse, abnormal wear & tear, accident, or environment issues. | CM |
| EF | Evident failure, i.e., a problem that can be detected, but was not reported by the user, without running any special tests or using specialized tester. | SM |
| SIF | Service-induced failure, i.e., caused by CM or SM that was not properlycompleted or a part that was replaced and failed prematurely (“infant mortality”). | CM |
| HF | Hidden failure, i.e., a problem that could not be detected by the user under normal circumstances, unless running a special test or using specializedtester. | SM |
| PF | Potential failure, i.e., failure is either about to occur or in the process ofoccurring but has not yet caused equipment to stop working or problems to patients or users. | SM |
| PPF | Preventable and predictable failure, typically caused by wear and tear that can be predicted or detected. | CM |
Table 2: Failure Cause Codes (FCCs). The rightmost column shows in which types of maintenance activity (SM or CM) the FCC can be used.
3.2.2 Reliability Evaluation Using EBM
First, collect all the service records with the following FCCs: service induced failure (SIF), hidden failure (HF), potential failure (PF) and preventable & predictable failure (PPF) found. Within each of these 4 FCCs, determine the number of equipment groups (i.e., same brand and model, and similar ages, utilization location and intensity, and users). Next, look for the equipment groups with unusually high number of FCCs, especially PPFs.
For these equipment groups, determine the underlying cause like “unsafe acts” (or “active failures”) committed by individual staff (employed by hospital, OEM, or third party), e.g., lapses or slips (individual) and “latent conditions” created by the organization due to oversight or deliberate violation of regulations, codes, or standards. If >50% of the FCCs are due to “latent conditions,” then determine whether it is caused by the adoption of a specific maintenance strategy. If so, revise it.
3.3 Use of EBM Evaluations #
Results of the safety and reliability evaluations should be used to revise and refine SM and CM strategies, i.e., to determine appropriate corrective and preventive actions that will not only correct but also prevent similar failures in the future in the maintenance of those equipment groups but also in others, if needed.
For “unsafe acts” (or “active failures”) committed by individual staff use one or more of the following actions: training, revision of work instructions, and disciplinary actions.
For “latent conditions” created by the organization use one or more of the following actions: revision of SM/CM strategies (procedures, frequencies, work instructions, etc.), and supervision of in-house and external service staff
4 Discussion & Conclusions #
4.1 EBM Limitations #
While EBM tries to imitate the methods of EB Medicine, there are some fundamental differences:
- Equipment is designed by people and, thus, is less complex and better understood than human beings
- Equipment does not suffer from psychosomatic effects, so double-blind approach is not needed
- Cannot blindfold CE professionals when performing service
In essence, it is not possible to conduct “double-blind randomized clinical trials” (RCT) like drug testing. EBM is more analogous to “cohort studies” in which the outcomes of two groups of patients are compared, one having received a certain therapy while the other did not.
4.2 Lessons Learned #
Data analysis show SIF is very rare [5]. Most maintenance errors are caused by active failures (human) instead of latent conditions (maintenance strategy). Thus, there is no reason to follow OEM maintenance recommendations, as these are typically overburdensome due to their desire to protect themselves against lawsuits. True PM is becoming obsolete with technology advance and will not provide job security. Artificial Intelligence (AI) and Machine Learning (ML) will soon allow predictive maintenance and, thus, reducing significantly CMs. Finally, relying solely on MTBF is misleading as many failures are not predictable or preventable. Should focus only on MTBF related to failures caused by maintenance activities.
4.3 How can EBM improve equipment maintenance? #
Why should CE professionals perform SM if it does not reduce failure or increase safety? For example, routine electrical safety tests (ESTs) have been abolished in the USA. Therefore, there is no reason to spend limited resources (time, material, money, etc.) on unnecessary maintenance activities. Instead, CE departments should reallocate its limited resources to:
- Help plan and select better equipment before purchase
- Help users to understand and use better the equipment
- Help users to understand and take better care of the equipment
- Help to determine when equipment needs to be replaced
- Help to investigate patient incidents related to medical equipment
- Address recalls promptly to reduce risks to patients and users
- Address cybersecurity issues presented by equipment
The results of adopting EBM in the USA have been replicated in Italy [6]. Other countries are also starting to adopt it although their results have not yet been published.
For over 30 years, the Fennigkoh & Smith model was adopted for maintenance of medical equipment at the American University of Beirut Medical Center (AUBMC). During this period of time, a failure coding system more elaborate than the EBM method described above was used. Although the data thus obtained helped to improve the maintenance strategy, it was more labor intensive and did not allow evaluation of safety and reliability as easily as the EBM method describe above.
The main challenges lying ahead in getting the full benefit of this system in our MENA region are:
- Willingness to adopt this method and share information across different institutions.
- Marketing the advantages of applying this method within each institution to the several key stakeholders.
- Convincing JCI and national accreditation bodies of the advantages of this approach instead of using the OEM recommendations and electrical safety checks.
References #
- “Evidence based medicine—an oral history”, British Med Journal. Available at https://www.bmj.com/content/348/bmj.g371. Accessed August 10, 2021. (2014).
- Wang B. Evidence-Based Maintenance of Medical Equipment: An Outcome-Based Method of Keeping Medical Equipment Safe and Reliable, BSI, Cornelius NC (2019).
- Fennigkoh, L. & Smith, B. “Clinical equipment management”. Plant, Technology and Safety Management,Volume 2, pp. 5-14. (1989).
- Moubray, J. Reliability-centered Maintenance. Second ed., Industrial Press, Inc. New York NY. (1997).
- Wang, B, Rui, T & Balar, S. “An estimate of patient incidents caused by medical equipment maintenance omissions”. Biomed Instrum Technol, Volume 47, pp. 84-91. (2013).
- Iadanza E, Gonneelli V, Satta F & Gherardelli M. “Evidence-based medical equipment management: A convenient implementation”. Med & Biol Eng & Comp, 57:2215-2230 (2019).







